The UPE Model EM1920 cup-shaped respirator consists of a semi-rigid inner-shell filter media and a cover-web. It covers the nose and mouth of the wearer and is held in place by two synthetic elastic headbands, conforming to the curvature of the wearer’s nose with a malleable aluminum nose-clip.


UPE intends to manufacture American-Made N95 Respirator Masks (“N95s”) in Great Falls, Montana using its own manufactured Polypropylene (“PP”) Nonwoven Fiber Fabric, also referred to as PP Melt Blown Fiber Media (“MBFM”), which is the main protective material used in the manufacturing of N95s.
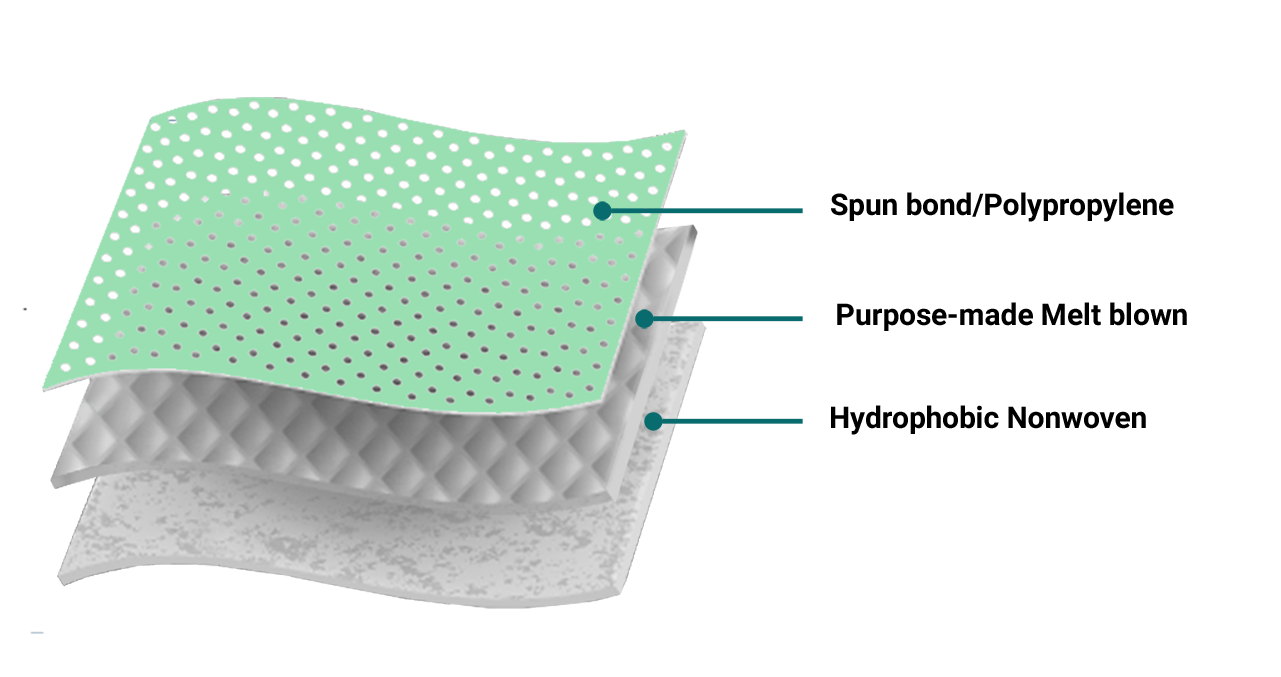
Spunbond fabrics are produced by depositing extruded, spun filaments onto a collecting belt in a uniform random manner followed by bonding the fibers. The fibers are separated during the web laying process by air jets or electrostatic charges. The collecting surface is usually perforated to prevent the air stream from deflecting and carrying the fibers in an uncontrolled manner.
Melt-blown process is a one-step process in which high-velocity air blows a molten thermoplastic resin from an extruder die tip onto a conveyor or take-up screen to form a fine fibrous and self-bonding web.
This type of material resists water penetration with extremely low absorbency and high stability.
Hydrophobic Material Characteristics:
High strength
Antibacterial
Soft
Lightweight
Water-repellent
EM Mask N95 Respirator Mask
Specifications
Features
Advantages
Materials
Use limitations
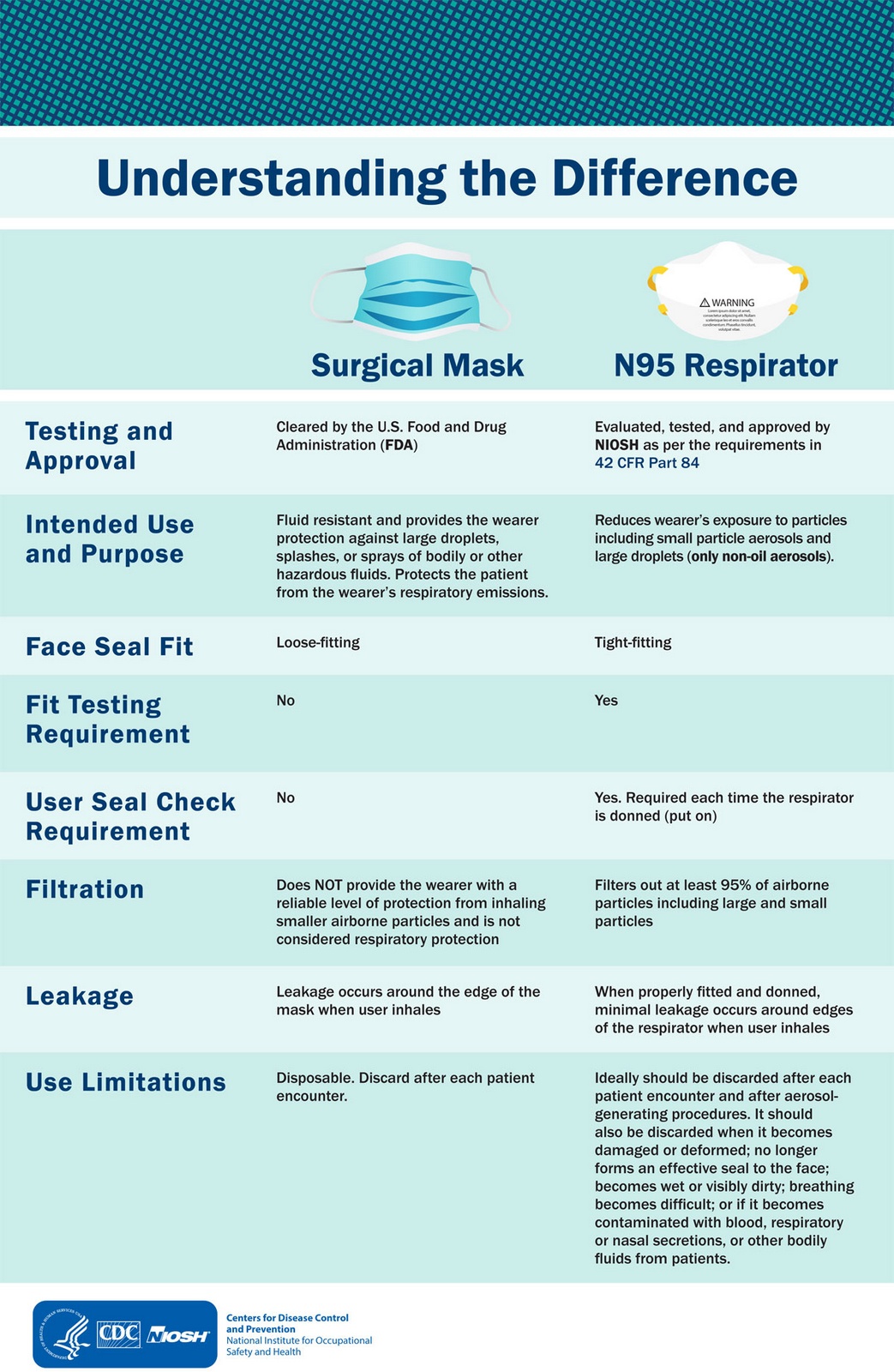
Center for Disease Control (“CDC”) guidelines
The Food and Drug Administration (“FDA”)


| Component | Weight (%) | |
| A l | 96.7 - 99 | |
| Cu | 0.05 - 0 .2 | |
| Mn | Max 0 .7 | |
| Other,each | Max 0.05 | |
| Other,total | Max 0 .15 | |
| Si | Max 0 .6 | |
| Zn | Max 0 .1 |



Do not use in atmosphere whose oxygen content is less than 19.5%. Do not use on atmosphere in wich the contaminant is IDLH concentration (Inmediately dangerous to life and health). (Spray paint, oiled aerosol, gas, organic steam and casting smoke)
If the respirator becomes damaged, soiled, or breathing becomes difficult, leave the contaminated area and replace and refit the new respirator.
*FDA and NIOSH approval pending

The Food and Drug Administration (“FDA”) is the regulatory body that certifies the N95 Particulate Respiratory and surgical Mask, also referred to a “Particulate Respirator”.
The UPE Model EM1920 cup-shaped respirator consists of a semi-rigid inner-shell filter media and a cover-web. It covers the nose and mouth of the wearer and is held in place by two synthetic elastic headbands, conforming to the curvature of the wearer’s nose with a malleable aluminum nose-clip.
The Classification Name: UPE Model EM1920 – Surgical Apparel, as described in 21 CFR 878.4040
The intended use meets the Center for Disease Control (“CDC”) guidelines for Tuberculosis (“TB”) exposure control. The filter efficiency level of 95% or greater against particulate aerosols free of oil (Type N95 respirator), minimizes wearer exposure to certain airborne particles in a size range of 0.10 to 10.0 microns (“µm”), such as those generated by electrocautery, laser and other powered medical instruments.
It is designed to be fluid resistant to splash, spatter of blood and body fluids and other potentially hazardous biomaterials. It provides greater than 99% Bacterial Filtration Efficiency* to exhaled wearer generated microorganisms (*as determined by the modified Greene and Vesley test method). No new technological characteristics are used in the UPE Model EM1920.


Filtration Efficiency
The UPE Model EM1920 meets the National Institute for Occupational Safety and Health (“NIOSH”), a federal government regulatory agency, required sodium chloride test with particles having a count median diameter of 0.055 µm to 0.095 µm and an aerodynamic diameter of 0.3 µm; at no time can the efficiency drop below 95%.
Fluid Resistance
The UPE Model EM1920 was challenged with 100 ml (+/-) 1 ml for up to 24 hours; no fluid penetration was observed.
Multiple Sized Particles Penetration Test – The UPE Model EM1920 was challenged with particles of multiple sizes, having an aerodynamic diameter range of 0.10 µm to 10.1 µm; the filter efficiency level was greater than 99%.
Bacterial Filtration Efficiency
The UPE Model EM1920 was tested using the modified Greene and Vesley procedure; filtration efficiency was greater than 99%.
Face Fit
The UPE Model EM1920 was tested using a qualitative fit test; face seal leakage was less than 10%.
Ease of Breathing
The UPE Model EM1920 meets the requirements of the NIOSH airflow resistance test which requires initial resistance (inhalation) to be less than 35mmH2O.
Conclusion
The UPE Model EM1920 non- clinical test results, when compared with data available and/or claims made on any predicate devices, demonstrate that the UPE Model EM1920 is as safe and effective as any predicate devices and performs as well as the predicate devices used in these experiments.
Many tasks performed by healthcare workers – such as patient intake and non-emergency patient evaluation – pose little risk of generating high-pressure streams of liquid and are not considered surgical procedures. For workers performing such tasks, a primary potential hazard to consider is airborne droplet containing viruses and bacteria, such as those generated by coughs and sneezes, which can be effectively filtered by a properly selected and worn N95 respirator.
Therefore, if a healthcare facility is prioritizing respirator use – due to limited supply during a health emergency – they may want to consider prioritizing use of surgical N95 respirators for those healthcare workers requiring respiratory protection while performing surgery or other tasks that may expose them to high pressure streams of bodily fluid.



| DESCRIPTION | STANDARD N95 RESPIRATOR |
UPE N95 RESPIRATOR MODEL EM1920 | SURGICAL N95 RESPIRATOR |
| Designed to help protect the wearer from exposure to airborne particles (e.g. Dust, mist, fumes, fibers, and bioaerosols, such viruses and bacteria) | YES | YES | YES |
| Designed to fit tightly to the face and create a seal between the user’s face and the respirator | YES | YES | YES |
| Meets NIOSH 42 CFR 84 N95 requirements for a minimum 95% filtration efficiency against solid and liquid aerosols that do not contain oil | YES | YES | YES |
| Cleared by the U.S. FDA as a surgical mask | NO | YES | YES |
| Not made with natural rubber latex | YES | YES | YES |
| Fluid Resistant - Meets ASTM Test Method F1862 “Resistance of Medical Face Masks to Penetration by Synthetic Blood” which determines the mask’s resistance to synthetic blood directed at it under varying high pressures.1 | NO | 120 mm Hg |
160 mm Hg |
| 1) ASTM F1862 is a standard test method for resistance of medical face masks to penetration by synthetic blood. This test is required because during certain medical procedures, a blood vessel may occasionally be punctured, resulting in a high-velocity stream of blood impacting a protective medical face mask. The test procedure specifies that a mask or respirator is conditioned in a high-humidity environment to simulate human use and is placed on a test holder. Synthetic blood (2cc) is shot horizontally at the mask at a distance of 30 cm (12 inches). | Surgical masks and respirators are tested on a pass/fail basis at three velocities corresponding to the range of human blood pressure (80, 120, and 160 mmHg). The inside of the mask is then inspected to see if any synthetic blood has penetrated to the inside of the face mask. Fluid resistance according to this test method is when the device passes at any level. 2) For more info visit the Frequently Asked Questions about Personal Protective Equipment section U.S. Centers for Disease Control and Prevention |
This chart demonstrates some key similarities and differences between three respirator models. The N95 respirator (featured) is a standard model, while the EM1920 and the surgical model (featured) are both surgical N95 respirators. |